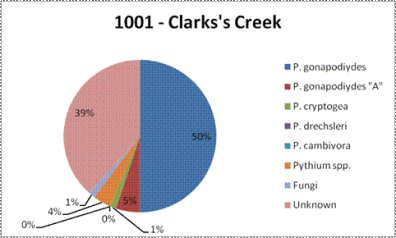
Oomycetes are fungus-like organisms found in marine, freshwater, and terrestrial environments. Some, such as Phytophthora, Pythium, and Saprolegnia, are parasites of plants and animals. DNA sequence data has revealed that these organisms are not fungi, but are more closely related to brown algae and diatoms. We will be “fishing” for these organisms by using leaf baits from various plant species.
Objectives
- Is there a relationship between host bait material and Oomycete species isolated?
- Riparian vs forest plants – are riparian plants resistant to infection?
- Has P. ramorum made it to Clarks Creek?
Each group will choose a plant species to use for bait. Pick fully expanded leaves that are relatively free of spots or other signs of infection. Punch out 20 leaf discs and put in bottle. Include one intact leaf. Put leaf baits in bottle of creek water and cap tightly. Rest bottle on its side for incubation.
After incubation leaf baits will be cultured on selective media for Oomycetes, transferred to pure culture, and identified using DNA sequencing.